Thanks to strict pollution norms enforced around the globe, catalytic converters have become mandatory. That’s because a catalytic converter can change highly noxious gases into agreeable compounds due to the clever usage of precious metals and chemistry.
Platinum, rhodium, and palladium aid a chemical reaction by working as a catalyst, crudely explained as an accelerant.
Converting Poisons to Manageable Compounds
These converters combine breathable oxygen, poisonous carbon monoxide, ozone-ailing unburned hydrocarbons, and harmful nitrogen oxides into manageable compounds. Conversely, these compounds are water, nitrogen, and carbon dioxide, a gas that we also emit but that’s also one of the leading greenhouse gases.
This choice was done to clear out cities that host hundreds of thousands of motorized vehicles, preferring to keep the citizen’s lungs free of diseases and the cities free of dangerous smog. A compromise, if you will, and one that humanity had to implement.
A French invention, the catalytic converter was designed by a mechanical engineer named Eugene Houdry in the mid-1950s, to mount them on gasoline engines. His reasoning was fueled by concerns regarding chimney and exhaust smoke and how they affect air pollution.
Consequently, his first-ever catalytic converter was mounted on chimneys, followed by forklifts that run on unleaded gasoline, and eventually regular cars (1).
See Also – What Causes White Exhaust Smoke?
Managing Harmful Compounds
When exhaust gases exit out of each cylinder they meet the exhaust headers, and thus the tailpipe. As such, the catalytic converter is mounted on the exhaust, as close as it can be to the engine.
These exhaust gases are exactly the harmful compounds explained above: carbon monoxide, unburned hydrocarbons, unburned or partially burned fuels, and nitrogen oxides.
When these gases enter the catalytic converter, they are burned further and suffer an oxidation process that must occur in split seconds. Otherwise, these pollutants would just spill out the tailpipe. Moreover, this oxidizing reaction would normally need a bit of time for it to take place, and this is where the catalyzer comes in (2).
Chemical Reactions
In the form of platinum, rhodium, and palladium, this catalyzer gives the oxidation reaction a push to facilitate the reaction process. This works since the compounds that we want to create, such as water or carbon dioxide, are more stable, and all these atoms would prefer taking the more stable form.
Given time, these reactions can occur naturally, but that would take too long. Such an item you might be familiar with is hydrogen peroxide, used to treat and disinfect wounds. If you were to leave a bottle of hydrogen peroxide with the cap off, it would rather transform into water and oxygen than stay hydrogen peroxide.
However, this process would take a while. Therefore, in our case, we are burning the harmful products to have the reactions happen faster, and even so, that’s not fast enough.
As such, we also have these reactions occur around these precious metals, seeing how they allow them to occur with less energy. But that’s not all, these metals also aid a reduction reaction that takes existing compounds, in our case nitrogen oxides, and breaks them down into nitrogen and oxygen (3).
Catalytic Converter Design
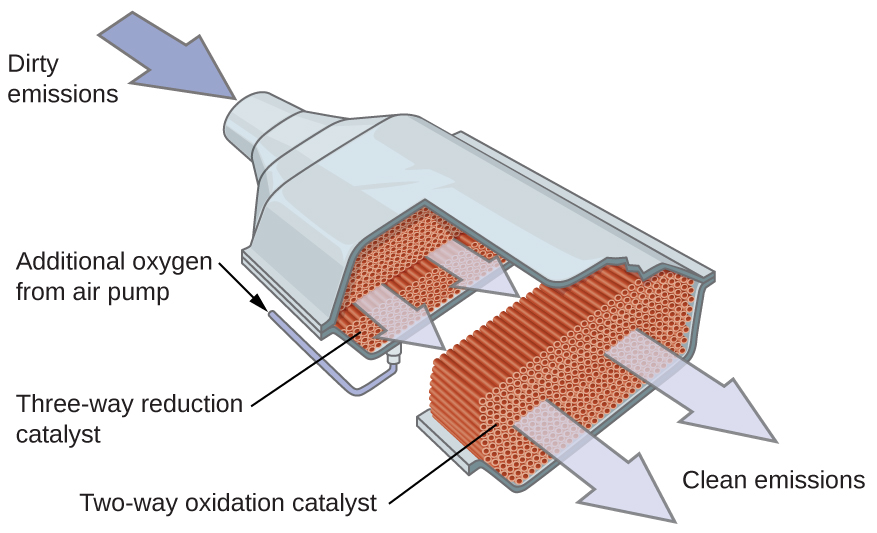
A catalytic converter is shaped like a squashed barrel, and if cut across, its section would show a very fine grid. That grid helps due to a myriad of factors. A grid has plenty of surface area, allowing the fast-moving air to get hotter faster.
Moreover, a honeycomb is easier to heat up and cool down, which is paramount for the proper working of a catalytic converter. Also, this grid allows us to split these noble metals evenly across the converter, making sure each reaction takes place as close to a catalyst as possible.
CC Temperature Threshold
However, a catalytic converter is quite finicky, considering the temperature inside must be maintained above a certain temperature for it to work properly, and the air-fuel ratio must meet a certain threshold. Without these, a catalytic converter is nothing but a choke on the exhaust.
These reduction-oxidation reactions (called redox reactions) are borderline effective at around 700-degrees Fahrenheit, but a faulty converter or a car that was left running and standing still for hours can reach over 1400F.
Anything below 700F would dictate that the reactions happen too slowly, and consequently, allow a myriad of pollutants into the atmosphere. Therefore, getting the catalytic converter as fast as possible up to the required temperature is paramount for an automotive engineer that works with engine maps.
Moreover, the air-fuel ratio must be maintained around its stoichiometric value, which should be around 14.7 parts of air to 1 part of the fuel for gasoline. This value is quite strict, only allowing a deviation of 3-percent over/under.
Otherwise, the engine will produce too much of one pollutant, and as a result, it won’t have all the necessary components to split nicely.
You can think of an air-fuel ratio as a Lambda value with the stoichiometric value of 1, calculated as the air used compared to air that should have been used to have the value as stoichiometric. If they are the same amount, the amount is stoichiometric, if there is more air used, the mixture is lean in fuel, and if less air is used, the mixture is rich in fuel.
Oxygen Sensors…Lambda Sensors
Oxygen sensors are commonly called Lambda sensors. They are paramount to the engine’s air-fuel ratio. They work by measuring the oxygen around the catalytic converter and return a value after some complex math. A stoichiometric ratio indicates that the fuel burned all the available oxygen, and consequently, the Lambda probe can easily find out if that was the case.
If it was not the case, it can send a command to the ECU and change the mixture so it gets as close to 1 as it can. The lambda probes also work at certain temperatures, around 600F. Moreover, seeing how the catalytic converter only works around a Lambda value of 1, a catalytic converter cannot work without Lambda probes, since you can’t control that value without them (4).
Catalytic converters now use only redox reactions, meaning that we also take care of nitrogen oxides. These are called three-way catalytic converters, and they were not the norm. Before them, we only used two-way catalytic converters that only oxidized hydrocarbons and carbon monoxide, but those are starting to become a rarity nowadays.
Missing or Damaged CC
If a catalytic converter is nonfunctional, cut, stolen, or punctured, then the engine will develop a myriad of issues. A clogged catalytic converter is basically clogging your entire exhaust, resulting in a significant loss of power or stability.
If the catalytic converter has problems, you will face big issues with fuel efficiency, because the Lambda probes will change the air-fuel ratio into nonsense.
If a Lambda probe is faulty, you can also enjoy poor fuel efficiency or low power, while also most likely ruining your catalytic converter in the process. Moreover, a bad converter can certainly translate into failed emission tests.
A check engine light can surely be from a problematic catalytic converter or a faulty lambda probe considering how a check engine light usually indicates a problem with the car’s antipollution systems. A punctured converter can allow extra air inside, resulting in noise, poor reaction efficiency, to a noticeable change in fuel economy and power characteristics.
Mitigating Climate Change
Even if not perfect, catalytic converters have helped massively with our fight regarding global warming. Cutting them and removing them is common, but only pursue that if you truly want to and know what you are getting into.
Bibliography
- Universal Tehnical Institute. What Is a Catalytic Converter and What Does It Do? [Online] January 06, 2021. [Cited: January 15, 2022.].
- United States Environmental Protection Agency. Basic Information about Carbon Monoxide (CO) Outdoor Air Pollution. [Online] June 07, 2021. [Cited: January 15, 2022.].
- Hamers, Laurel. Explainer: What is a catalyst? [Online] February 27, 2017. [Cited: January 15, 2022.].
- Hella. Lambda sensor—cause of failure, testing and troubleshooting. [Online] [Cited: January 15, 2022.].
Photo Attribution
- Ahanix1989 at English Wikipedia, Public domain, via Wikimedia Commons
- OpenStax, CC BY 4.0, via Wikimedia Commons
See Also – What is Regenerative Braking?
- What Are Radial Tires? - Aug 22, 2023
- What is the Coefficient of Drag? - Jun 7, 2022
- 6 Signs of Steering Rack Failure - May 27, 2022